Gasoline is a vital fuel source powering countless vehicles worldwide. However, its volatile nature presents challenges, particularly concerning evaporation. This article delves into the fascinating process of does gas evaporate in heat, exploring the scientific principles behind it and its practical implications. We’ll examine how heat influences gasoline’s evaporation rate, delve into the composition of this fuel, and understand why certain factors accelerate this process.
Gasoline Evaporation
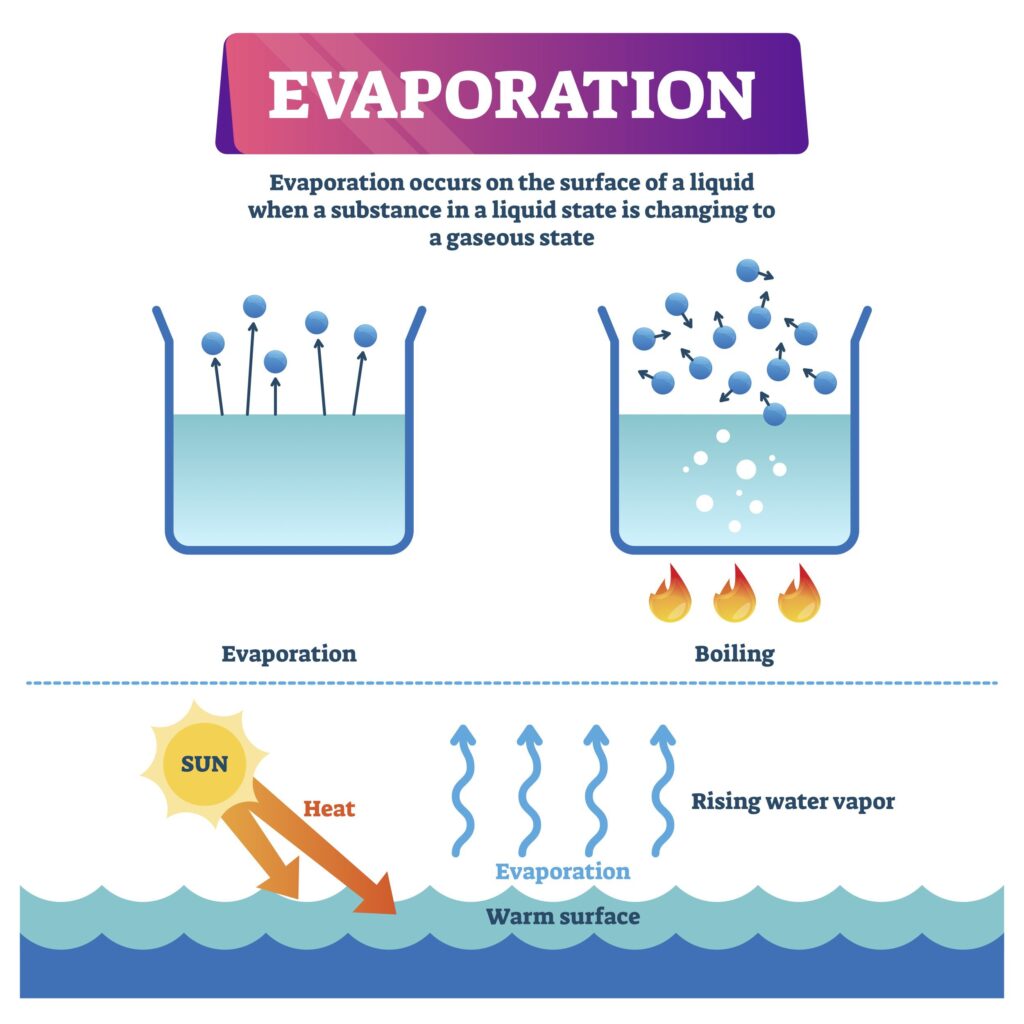
Gasoline evaporation is a natural phenomenon where liquid gasoline transforms into gaseous vapor. This occurs when gasoline molecules gain enough energy to overcome the intermolecular forces holding them together in the liquid state. As these molecules escape into the atmosphere, they contribute to air pollution and can pose safety hazards if not managed properly.
The rate of gasoline evaporation depends on several factors, with temperature being a primary influencer. Warmer temperatures accelerate the process significantly, as heat provides more energy for gasoline molecules to break free from their liquid bonds. This is why fuel tanks tend to lose more gasoline during hot weather compared to cooler periods.
Heat and Kinetic Energy
Heat plays a crucial role in does gas evaporate in heat by influencing the kinetic energy of gasoline molecules. Kinetic energy refers to the energy possessed by objects due to their motion. When heat is applied to gasoline, its molecules absorb this energy and begin to move faster and with greater intensity.
Increased molecular movement weakens the intermolecular forces holding them together in the liquid state. Eventually, some molecules gain enough kinetic energy to overcome these forces entirely and escape into the atmosphere as vapor. This process is known as evaporation.
Factors Affecting Kinetic Energy
Several factors can influence the kinetic energy of gasoline molecules, including:
Temperature: Higher temperatures directly translate to increased molecular movement and higher kinetic energy.
Pressure: Lower atmospheric pressure allows for easier escape of gasoline molecules into the atmosphere, increasing evaporation rates.
Surface Area: A larger surface area exposed to air allows for more molecules to come in contact with the surrounding environment, promoting evaporation.
Impact of Temperature on Evaporation
The relationship between temperature and gasoline evaporation is directly proportional. As temperatures rise, the rate of evaporation increases significantly. This is because higher temperatures provide gasoline molecules with more kinetic energy, enabling them to break free from their liquid bonds more readily.
On hot days, for example, gasoline stored in open containers or vehicles can evaporate at a much faster rate compared to cooler conditions. This phenomenon highlights the importance of proper fuel storage and handling practices, especially during periods of extreme heat.
Volatile Liquids
Gasoline is classified as a volatile liquid due to its tendency to evaporate readily at relatively low temperatures. Volatility refers to the ease with which a substance transitions from a liquid to a gaseous state.
Volatile liquids have weak intermolecular forces holding their molecules together, allowing them to escape into the atmosphere more easily. This characteristic makes gasoline susceptible to evaporation and contributes to air pollution concerns.
Hydrocarbons
Gasoline is primarily composed of hydrocarbons, organic compounds consisting solely of hydrogen and carbon atoms. These hydrocarbons vary in size and structure, contributing to gasoline’s complex composition and its diverse properties.
The hydrocarbon chains in gasoline molecules are relatively short, allowing for easier vaporization. This characteristic further contributes to gasoline’s volatility and its susceptibility to evaporation under various conditions.
Conclusion
Understanding the process of does gas evaporate in heat is crucial for comprehending the behavior of gasoline and mitigating potential risks associated with its volatile nature. Heat significantly accelerates gasoline evaporation by increasing the kinetic energy of its molecules, weakening intermolecular forces, and facilitating their transition into the gaseous state.
Proper fuel storage practices, awareness of environmental impacts, and responsible handling are essential to minimize the negative consequences of gasoline evaporation. By understanding the science behind this phenomenon, we can make informed decisions regarding fuel usage and contribute to a safer and more sustainable environment.