The universe around us, in all its complexity and diversity, is built from incredibly tiny particles called atoms. These fundamental units of matter are the foundation upon which everything we see, touch, and experience is constructed. While atoms can undergo transformations through various processes, their core structure remains remarkably stable, leading to the intriguing question: do atoms last forever?
This article delves into the fascinating world of atoms, exploring their nature, structure, and the processes that influence them. We’ll examine how atoms participate in chemical reactions and nuclear processes, ultimately shedding light on the enduring nature of these fundamental building blocks and their role in the universe’s continuous existence.
What Are Atoms?
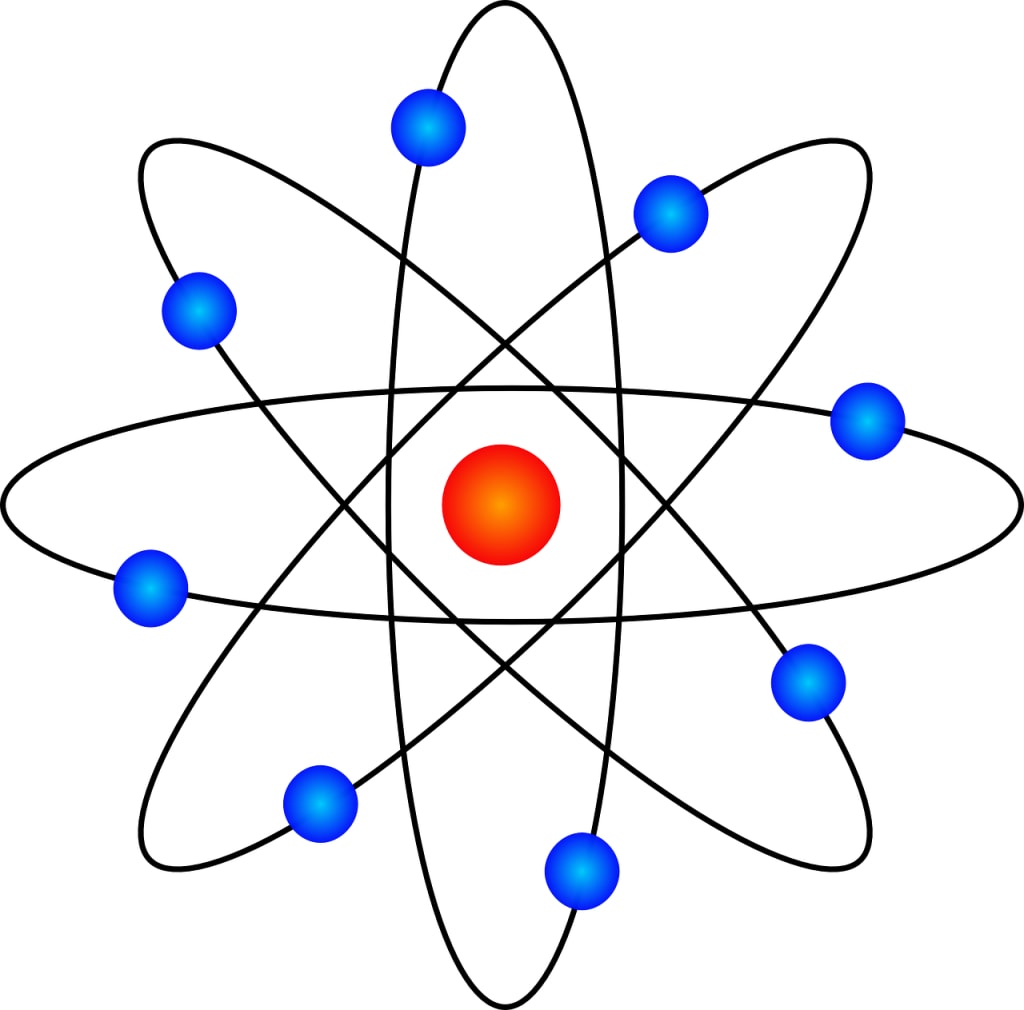
Atoms are the smallest unit of an element that retains the chemical properties of that element. They are incredibly small, with a diameter typically measured in picometers (one trillionth of a meter). Despite their minuscule size, atoms possess immense power and complexity. Each atom consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The nucleus itself is composed of positively charged protons and neutral neutrons.
The number of protons within an atom’s nucleus defines its atomic number, which determines the element to which it belongs. For example, all carbon atoms have six protons, while oxygen atoms have eight. Atoms of the same element can have different numbers of neutrons, resulting in isotopes. Isotopes are variations of an element with the same atomic number but differing mass numbers due to the varying neutron count.
Atomic Structure

The structure of an atom is a marvel of intricate organization. At its heart lies the nucleus, a tiny region containing almost all of the atom’s mass. Protons and neutrons, collectively known as nucleons, are tightly bound together within the nucleus by the strong nuclear force, one of the fundamental forces of nature. The protons within the nucleus carry a positive electric charge, while neutrons have no charge.
Surrounding the nucleus is a vast expanse of space occupied by electrons. These negatively charged particles orbit the nucleus in specific energy levels or shells. Each shell can accommodate a limited number of electrons, and the arrangement of electrons within these shells determines an atom’s chemical properties and how it interacts with other atoms. The outermost shell, known as the valence shell, plays a crucial role in chemical bonding.
Electron Configuration
The arrangement of electrons within an atom’s energy levels is described by its electron configuration. This configuration dictates how an atom will interact with other atoms to form bonds and create molecules. Electrons occupy orbitals, which are regions of space around the nucleus where an electron is most likely to be found. Each orbital can hold a specific number of electrons, and the order in which orbitals are filled follows specific rules known as Aufbau principle, Hund’s rule, and Pauli exclusion principle.
Chemical Reactions and Atoms
Atoms are constantly interacting with each other through chemical reactions. These reactions involve the breaking and forming of bonds between atoms, resulting in the rearrangement of atoms into new molecules or compounds. Chemical reactions are driven by the desire of atoms to achieve a stable electron configuration, often by sharing or transferring electrons with other atoms.
Types of Chemical Reactions
There are various types of chemical reactions, including:
* Synthesis reactions: Two or more reactants combine to form a single product.
* Decomposition reactions: A single reactant breaks down into two or more products.
* Single displacement reactions: One element replaces another element in a compound.
* Double displacement reactions: The positive and negative ions of two reactants switch places, forming two new compounds.
During chemical reactions, the atoms themselves are not destroyed or created; they simply rearrange to form new combinations. The total number of atoms of each element remains constant before and after the reaction, adhering to the law of conservation of mass.
Nuclear Processes and Atoms
While chemical reactions involve changes in electron configurations, nuclear processes affect the very nucleus of an atom. These processes involve changes in the number of protons or neutrons within the nucleus, leading to the transformation of one element into another.
Radioactive Decay
Radioactive decay is a spontaneous process where unstable atomic nuclei emit particles and energy to become more stable. Different types of radioactive decay include alpha decay, beta decay, and gamma decay. Each type involves the emission of different particles or energy, resulting in the transformation of the original nucleus into a new element.
Nuclear Fission
Nuclear fission is a process where a heavy atomic nucleus, such as uranium-235, is split into two or more lighter nuclei when bombarded with a neutron. This process releases a tremendous amount of energy, which is harnessed in nuclear power plants and weapons.
Nuclear Fusion
Nuclear fusion involves the combining of light atomic nuclei, such as hydrogen isotopes, to form a heavier nucleus, releasing vast amounts of energy. This process powers the sun and other stars.
The Enduring Nature of Atoms
Despite undergoing transformations through chemical reactions and nuclear processes, the fundamental nature of atoms remains remarkably stable. The core structure of an atom, consisting of protons and neutrons bound together in the nucleus, persists through these changes.
While individual atoms may be transformed or destroyed during nuclear processes, the total number of atoms in the universe remains constant. This principle is known as the law of conservation of mass-energy, which states that energy and mass can be converted into each other but cannot be created or destroyed.
Conclusion
Atoms are the fundamental building blocks of matter, possessing a remarkable stability and enduring nature. While they can undergo transformations through chemical reactions and nuclear processes, their core structure remains constant. The concept of do atoms last forever highlights the enduring nature of these tiny particles and their essential role in the universe’s continuous existence. From the smallest molecule to the largest star, atoms are the foundation upon which the vast tapestry of the cosmos is woven.