The interaction between gasoline and ice is a common question, often arising from the observation that gasoline remains liquid even in cold temperatures. While it’s true that gasoline has a low freezing point, this doesn’t automatically mean it can melt ice. Understanding the properties of both substances and the process of melting is crucial to answering this question accurately. This article will delve into the characteristics of gasoline and ice, explore the melting process, and ultimately determine whether does gasoline melt ice.
This article will first examine the key properties of gasoline, including its freezing point and composition. Next, we’ll discuss the scientific process of ice melting, focusing on the role of heat energy. We’ll then analyze the interaction between gasoline and ice, considering factors like heat capacity and temperature change. Finally, we’ll arrive at a conclusive answer to the question: does gasoline melt ice?.
Gasoline Properties
Gasoline is a complex mixture of hydrocarbons, primarily derived from crude oil. Its composition varies depending on the refining process and intended use. However, it generally consists of molecules with between 4 and 12 carbon atoms. This intricate structure contributes to gasoline’s characteristic flammability and volatility.
One crucial property of gasoline is its freezing point, which is typically around -47°C (-53°F). This relatively low freezing point allows gasoline to remain liquid even in cold weather conditions. However, it’s important to note that this doesn’t imply that gasoline possesses the ability to melt other substances.
Another significant characteristic of gasoline is its heat capacity. Heat capacity refers to the amount of heat energy required to raise the temperature of a substance by a specific degree. Gasoline has a relatively low heat capacity compared to many other materials, meaning it takes less energy to change its temperature.
Ice Melting Process
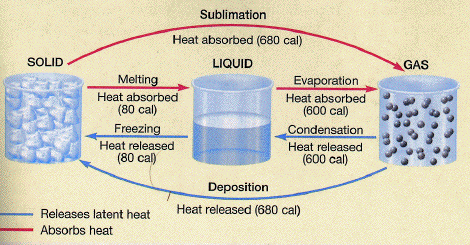
Ice melting is a physical change that involves the transition of water from its solid state to its liquid state. This process occurs when the ice absorbs enough heat energy to overcome the intermolecular forces holding the water molecules in a rigid crystalline structure.
The melting point of ice is 0°C (32°F) at standard atmospheric pressure. At this temperature, the absorbed heat energy breaks the bonds between water molecules, allowing them to move more freely and transition into a liquid state. The amount of heat energy required to melt a given mass of ice is known as its latent heat of fusion.
Factors Affecting Melting Rate
Several factors can influence the rate at which ice melts, including:
- Temperature: Higher temperatures accelerate the melting process by providing more heat energy to break the bonds between water molecules.
- Surface Area: Ice with a larger surface area exposed to heat will melt faster because there are more points of contact for heat transfer.
- Heat Transfer Mechanism: Conduction, convection, and radiation can all contribute to ice melting. Convection, involving the movement of heated fluids, is particularly effective in accelerating the process.
Heat Capacity and Temperature Change
Heat capacity plays a crucial role in determining how much temperature change occurs when heat energy is transferred between substances. A substance with a high heat capacity requires more heat energy to raise its temperature by a given amount compared to a substance with a low heat capacity.
Gasoline, as mentioned earlier, has a relatively low heat capacity. This means that it can absorb a significant amount of heat energy without experiencing a large temperature increase. Conversely, ice has a higher heat capacity than gasoline. Therefore, when gasoline comes into contact with ice, the heat transfer from gasoline to ice will be relatively small due to gasoline’s low heat capacity.
Gasoline vs. Ice Interaction
When gasoline and ice are brought together, heat energy will naturally flow from the warmer substance (gasoline) to the colder substance (ice). However, due to gasoline’s low heat capacity and the relatively high heat capacity of ice, the amount of heat transferred will be insufficient to raise the temperature of the ice significantly.
As a result, does gasoline melt ice? The answer is no. While gasoline remains liquid in cold temperatures, its inability to transfer sufficient heat energy prevents it from melting ice.
Conclusion
The interaction between gasoline and ice highlights the importance of understanding both the properties of substances and the principles of heat transfer. While gasoline’s low freezing point allows it to remain liquid even in cold conditions, its low heat capacity limits its ability to transfer enough heat energy to melt ice. Therefore, does gasoline melt ice? The answer is a definitive no.

![Pharmacy Near [Cross Streets] – Prescription & OTC Remedies](https://nodumbquestions.net/wp-content/uploads/pharmacy-near-cross-streets-prescription-otc-remedies-150x150.jpg)
