Ice cream, a universally adored frozen dessert, has tantalized taste buds for centuries. Its creamy texture and delightful flavors make it a summertime staple and a comforting treat year-round. But have you ever stopped to consider the scientific classification of this beloved confection? While its smooth consistency might lead you to believe otherwise, is ice cream a liquid or solid? The answer lies in understanding the intricate processes that transform a simple mixture into the frozen delight we know and love.
This article delves into the fascinating science behind ice cream, exploring its classification, composition, freezing process, texture, melting behavior, and ultimately revealing the truth about whether is icecream a liquid or a solid. We’ll unravel the mysteries of this frozen treat, shedding light on the factors that contribute to its unique characteristics.
Ice Cream Classification
From a scientific standpoint, is ice cream a liquid or solid? The answer is unequivocally solid. Despite its seemingly fluid nature when scooped, ice cream possesses the defining characteristics of a solid: it maintains a fixed shape and resists flow under normal conditions. The freezing process solidifies the mixture, transforming it into a state where molecules are tightly packed together, restricting their movement.
While ice cream can melt into a liquid state at warmer temperatures, this transformation is temporary. Upon cooling, it reverts back to its solid form, reaffirming its classification as a solid. This reversible transition between solid and liquid states is characteristic of many substances, including water, but ultimately, the inherent structure of ice cream defines it as a solid.
Composition of Ice Cream
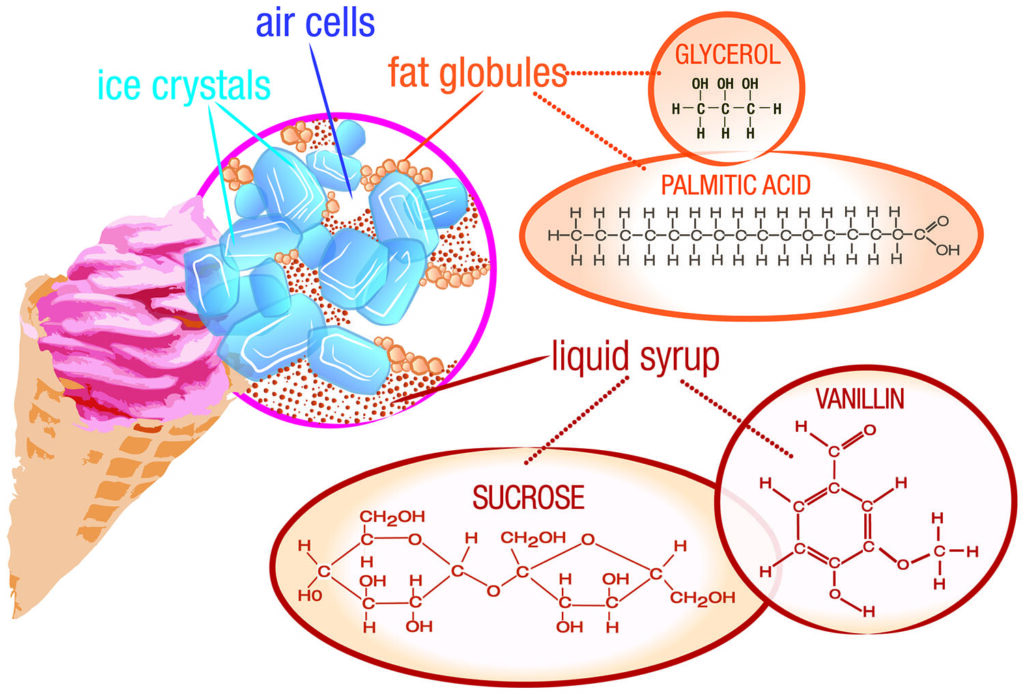
The delightful taste and texture of ice cream stem from its carefully crafted composition. The primary ingredients include:
- Dairy: Milk or cream provides the base for ice cream, contributing to its creamy richness and smooth mouthfeel. The fat content in dairy influences the final texture; higher fat content results in a richer, denser ice cream.
Sugar: Sugar not only sweetens the mixture but also plays a crucial role in lowering the freezing point of water. This prevents the formation of large ice crystals, ensuring a smoother texture.
Flavorings: A wide array of flavorings can be incorporated into ice cream, ranging from fruits and nuts to chocolate and caramel. These additions contribute to the diverse range of flavors and textures available.
- Air: During the churning process, air is incorporated into the mixture, creating a lighter and airier texture. The amount of air incorporated influences the density and volume of the final product.
Freezing Process
The transformation of ice cream from a liquid mixture to a solid treat involves a carefully controlled freezing process:
- Pasteurization: Milk and cream are heated to kill harmful bacteria, ensuring the safety and longevity of the finished product.
- Mixing: Ingredients are combined in precise proportions, creating a homogenous base for the ice cream.
Churning: The mixture is continuously agitated while being frozen, incorporating air and preventing large ice crystals from forming. This churning process creates the characteristic smooth texture of ice cream.
Hardening: Once churned, the ice cream is transferred to containers and further frozen until it reaches a solid state.
Texture and Scoopability
The unique texture of ice cream, its ability to be scooped effortlessly, stems from several factors:
- Fat Content: Higher fat content in dairy contributes to a smoother, richer texture. Fat molecules coat the ice crystals, preventing them from sticking together and creating a more cohesive mass.
Sugar Concentration: Sugar lowers the freezing point of water, inhibiting the formation of large ice crystals. This results in a finer texture with smaller ice crystals dispersed throughout the mixture.
Air Incorporation: The churning process incorporates air into the mixture, creating pockets that contribute to the lightness and scoopability of ice cream.
Melting Behavior
While ice cream is classified as a solid, it exhibits a fascinating melting behavior:
- Temperature Dependence: Ice cream melts at temperatures above its freezing point, typically around 0°C (32°F). The rate of melting depends on the surrounding temperature and the composition of the ice cream.
Phase Transition: Melting is a reversible phase transition where the solid ice cream transforms into a liquid state. During this process, the molecules gain energy and begin to move more freely, breaking the rigid structure of the solid.
Texture Changes: As ice cream melts, its texture changes from firm and scoopable to soft and runny. The smaller ice crystals gradually dissolve, resulting in a smoother consistency.
Conclusion
The beloved frozen treat we know as ice cream is scientifically classified as a solid, despite its smooth and scoopable texture. Understanding the intricate processes involved in its creation—from the careful selection of ingredients to the precise freezing process—reveals the fascinating science behind this delightful dessert. While it can melt into a liquid state at warmer temperatures, is ice cream a liquid? Ultimately, the inherent composition and structure of ice cream define it as a solid, solidifying its place in our hearts (and freezers) as a timeless treat.