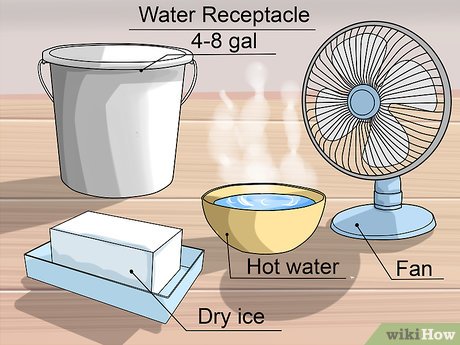
Beat the heat and transform your room into an oasis of coolness with the surprising power of dry ice. This unique substance offers a rapid and effective cooling solution, perfect for those sweltering summer days or when you need to quickly lower the temperature in a confined space. In this article, we’ll delve into the science behind dry ice cooling, explore its numerous benefits, and provide a comprehensive guide on how to safely cool your room using this fascinating substance.
Dry Ice Cooling Explained
Dry ice is the solid form of carbon dioxide (CO2). Unlike regular ice which melts into water, dry ice undergoes sublimation – a process where it transitions directly from a solid to a gas. This unique characteristic makes dry ice an incredibly efficient cooling agent. As dry ice sublimates, it absorbs significant amounts of heat from its surroundings, effectively lowering the temperature of the enclosed space.
The rapid absorption of heat during sublimation is what makes dry ice so effective for cooling. It’s able to chill a room much faster than traditional methods like fans or air conditioning, especially in smaller spaces. This makes it a popular choice for events, parties, and even scientific experiments where quick temperature reduction is essential.
Sublimation Process

The sublimation process of dry ice is fascinating to observe. When exposed to warmer temperatures, the solid CO2 molecules gain energy and break free from their rigid structure. Instead of melting into a liquid, they transition directly into gaseous CO2, which then disperses into the air. This phase change requires a significant amount of heat energy, effectively drawing heat away from the surrounding environment and causing a cooling effect.
The rate of sublimation depends on several factors, including the temperature difference between the dry ice and its surroundings, the surface area exposed, and the airflow present. In enclosed spaces with limited airflow, the sublimation process is slower as the CO2 gas has less room to escape. Conversely, in open areas with good ventilation, the sublimation rate increases significantly.
Benefits of Using Dry Ice
Dry ice offers several advantages over traditional cooling methods:
Rapid Cooling: As mentioned earlier, dry ice’s sublimation process allows for incredibly fast temperature reduction, making it ideal for situations requiring immediate cooling.
No Messy Water: Unlike regular ice which melts and leaves behind puddles, dry ice sublimates directly into gas, leaving no residue or mess. This makes it a convenient and clean option for various applications.
- Lower Temperatures: Dry ice can achieve significantly lower temperatures than traditional ice, reaching as low as -78.5°C (-109.3°F). This extreme cold is beneficial for specific applications like preserving biological samples or creating fog effects.
How to Cool a Room with Dry Ice Safely

While dry ice is an effective cooling agent, it’s crucial to handle it safely:
- Wear Gloves: Always wear insulated gloves when handling dry ice as direct contact can cause severe frostbite.
Ventilation: Ensure adequate ventilation in the room where you are using dry ice. The CO2 gas produced during sublimation can displace oxygen and pose a risk if not properly ventilated. Open windows or use fans to circulate air.
Avoid Direct Contact with Skin: Keep dry ice away from skin and eyes. If contact occurs, immediately flush the affected area with warm water for at least 15 minutes.
- Store Properly: Store dry ice in a well-ventilated container, such as a cooler lined with towels or cardboard to prevent direct contact with the container walls.
Do not use dry ice to cool a room by placing it directly on the floor or near flammable materials.
Temperature Reduction Results
The effectiveness of using dry ice to cool a room depends on several factors, including the size of the room, the initial temperature, and the amount of dry ice used. In general, you can expect a noticeable temperature drop within 30 minutes to an hour after introducing dry ice into a well-ventilated space.
For smaller rooms (around 100 square feet), using 5-10 pounds of dry ice can effectively lower the temperature by 10-15 degrees Fahrenheit. Larger rooms may require more dry ice, and it’s important to monitor the temperature and adjust accordingly. Remember that the cooling effect will gradually diminish as the dry ice sublimates.
Conclusion
Dry ice to cool a room offers a unique and effective solution for rapidly lowering temperatures in enclosed spaces. Its rapid sublimation process absorbs significant heat, making it ideal for various applications, from beating the summer heat to creating special effects. However, it’s crucial to handle dry ice safely by wearing gloves, ensuring proper ventilation, and avoiding direct contact with skin or flammable materials. By following these guidelines, you can harness the power of dry ice to create a refreshingly cool environment in your home or workspace.