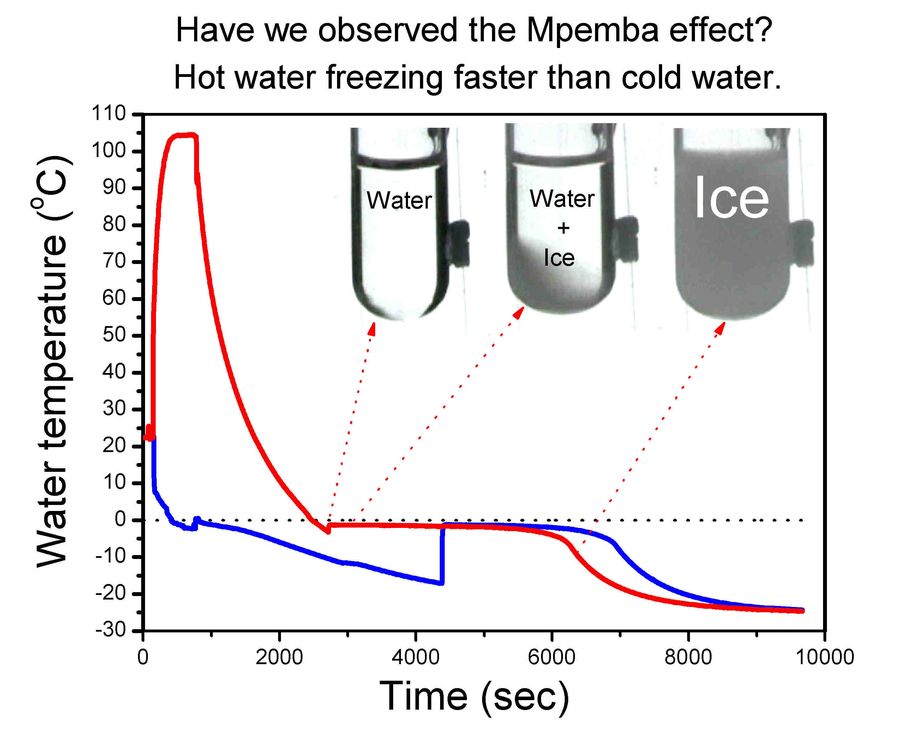
The world of science is full of intriguing phenomena that challenge our everyday understanding. One such phenomenon is the Mpemba effect, which suggests that under certain conditions, does salt water freeze faster than freshwater. This seemingly counterintuitive observation has puzzled scientists for decades, leading to a flurry of research and debate surrounding its potential explanations.
This article delves into the fascinating world of the Mpemba effect, exploring its history, various theories attempting to explain it, and the ongoing scientific research seeking to unravel this intriguing paradox. We’ll examine the differences in freezing rates between saltwater and freshwater, analyze the factors that might influence these rates, and ultimately shed light on the complexities surrounding this captivating scientific puzzle.
Mpemba Effect Explained
The Mpemba effect is named after Erasto Mpemba, a Tanzanian student who observed this phenomenon in the 1960s while preparing ice cream. He noticed that hot milk with sugar froze faster than cold milk, prompting him to investigate further. While his initial observation focused on sugary solutions, the term “Mpemba effect” has since come to encompass any instance where a hotter liquid freezes faster than a colder one under similar conditions.
This effect is not always observed and depends on various factors, including the starting temperature difference, container shape, and environmental conditions. However, when it does occur, it defies our intuitive understanding of thermodynamics, which suggests that colder liquids should freeze faster due to their lower initial energy levels.
Saltwater vs. Freshwater Freezing

The Mpemba effect is often observed when comparing saltwater and freshwater freezing rates. While freshwater typically freezes at 0°C (32°F), the presence of dissolved salts in saltwater lowers its freezing point. This means that saltwater needs to be cooled further before it reaches its freezing point compared to freshwater.
However, despite this initial temperature difference, some experiments have shown that saltwater can freeze faster than freshwater under specific conditions. This observation has fueled the debate surrounding the Mpemba effect and led scientists to explore various potential explanations for this seemingly paradoxical phenomenon.
Theories and Explanations
Several theories attempt to explain the Mpemba effect, each proposing different mechanisms that could lead to faster freezing in certain situations. Some of the most prominent theories include:
- Evaporation: Hotter liquids tend to evaporate more rapidly than colder ones. This evaporation can reduce the volume of liquid needing to freeze, potentially leading to a faster freezing time.
- Convection: Hotter liquids exhibit stronger convection currents, which can enhance heat transfer and accelerate cooling.
- Dissolved Salts: The presence of dissolved salts in saltwater can influence the freezing point and heat transfer properties of the solution, potentially contributing to faster freezing rates under certain conditions.
Scientific Research and Debate

The Mpemba effect has been a subject of ongoing scientific research for decades. Numerous studies have attempted to replicate and analyze this phenomenon, exploring various factors that might influence freezing rates. While some studies have provided evidence supporting the Mpemba effect, others have failed to reproduce it consistently. This inconsistency highlights the complexity of the phenomenon and the need for further investigation.
The debate surrounding the Mpemba effect continues within the scientific community, with researchers proposing new theories and refining existing ones. The lack of a definitive explanation has fueled further research, pushing scientists to delve deeper into the intricate world of thermodynamics and explore the complex interplay of factors that govern freezing processes.
Factors Influencing Freezing Rates
Numerous factors can influence the rate at which a liquid freezes, including:
- Initial Temperature: The starting temperature difference between two liquids significantly impacts their freezing times.
- Container Shape and Material: The shape and material of the container can affect heat transfer rates and influence freezing times.
- Environmental Conditions: Ambient temperature and air currents can impact the rate at which a liquid loses heat and freezes.
- Liquid Composition: The presence of dissolved substances, such as salts or sugars, can alter the freezing point and heat transfer properties of a liquid.
Conclusion
The Mpemba effect remains a captivating scientific puzzle, challenging our conventional understanding of thermodynamics. While numerous theories attempt to explain this phenomenon, a definitive explanation remains elusive. Ongoing research continues to explore the complex interplay of factors influencing freezing rates, shedding light on the intricate mechanisms behind this intriguing paradox. The quest to unravel the mysteries of the Mpemba effect highlights the boundless wonders of science and the constant pursuit of knowledge that drives scientific exploration.