Have you ever witnessed the peculiar sight of ice floating serenely on top of a body of cold water? This seemingly simple observation unveils a fascinating interplay between temperature and density, showcasing how frozen water defies gravity by remaining suspended above its liquid form. This article delves into the scientific principles behind this captivating phenomenon, exploring the unique properties of ice that enable it to float.
We’ll begin by examining the concepts of density and buoyancy, understanding how they govern the behavior of objects in fluids. Then, we’ll delve into the specific relationship between temperature and water density, revealing why ice is less dense than liquid water. Finally, we’ll explore the implications of this unique property for various cold water environments and its role in shaping our planet’s ecosystems.
Ice Density and Buoyancy
Density refers to the mass of a substance per unit volume. In simpler terms, it describes how tightly packed the molecules are within an object. Buoyancy, on the other hand, is the upward force exerted by a fluid that opposes the weight of an immersed object. This force arises from the pressure difference between the top and bottom surfaces of the submerged object.
When an object is placed in a fluid, it experiences both its own weight acting downwards and the buoyant force acting upwards. If the buoyant force exceeds the object’s weight, the object will float; if the weight is greater, it will sink. The density of both the object and the fluid play crucial roles in determining whether an object will float or sink.
The Science of Floating Ice

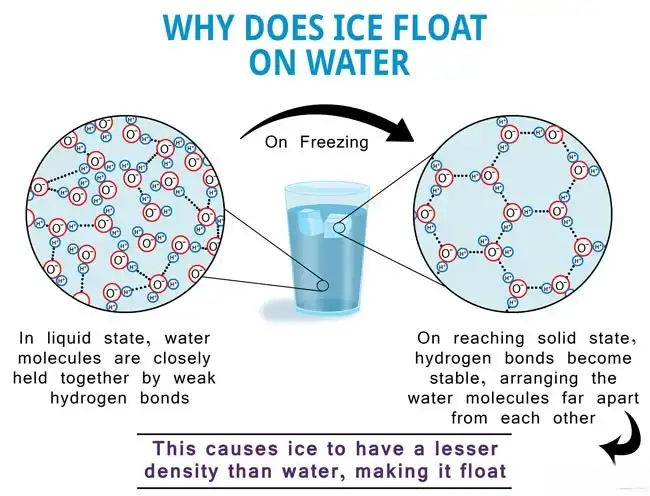
Ice floating on cold water is a testament to the unique properties of water. Unlike most substances, water becomes less dense as it freezes. This means that ice, with its tightly packed crystal structure, has a lower density than liquid water. As a result, when ice forms at the surface of a body of water, it displaces an amount of liquid water equal to its own weight.
This displacement creates an upward buoyant force that counteracts the downward pull of gravity, allowing the ice to remain afloat. The less dense ice acts as a barrier, insulating the underlying liquid water and preventing it from freezing solid. This phenomenon is crucial for aquatic life, as it allows bodies of water to remain liquid even during frigid temperatures.
Temperature and Water Density
The relationship between temperature and water density is complex and non-linear. Water reaches its maximum density at 4 degrees Celsius (39.2 degrees Fahrenheit). As water cools below this temperature, its density decreases due to the formation of a crystalline structure in the ice phase. This unique behavior stems from the hydrogen bonding between water molecules.
At higher temperatures, water molecules move freely and are more spread out, resulting in a higher density. However, as the temperature drops towards 4 degrees Celsius, the hydrogen bonds become more prominent, causing the molecules to arrange themselves into a less dense hexagonal lattice structure. This decrease in density continues as the water freezes completely, forming ice with a significantly lower density than liquid water.
Unique Properties of Ice

Ice possesses several unique properties that distinguish it from other solids and contribute to its ability to float. Firstly, its crystalline structure, characterized by a hexagonal lattice arrangement, creates numerous air pockets within the solid mass. These air pockets reduce the overall density of ice compared to liquid water.
Secondly, ice is less compressible than liquid water due to the rigid nature of its crystal structure. This means that ice occupies a larger volume for a given mass compared to liquid water. Finally, ice has a lower thermal conductivity than liquid water, meaning it conducts heat more slowly. This property plays a crucial role in insulating bodies of water and protecting aquatic life from extreme temperature fluctuations.
Ice in Cold Water Environments
The ability of ice to float has profound implications for various cold water environments. In lakes and oceans, floating ice acts as an insulator, preventing the underlying water from freezing solid and allowing aquatic life to survive during winter months. This phenomenon is crucial for maintaining biodiversity and supporting complex food webs in these ecosystems.
Furthermore, floating ice plays a vital role in regulating global climate patterns. The reflective surface of ice reflects sunlight back into space, helping to cool the planet. Conversely, melting ice absorbs solar radiation, contributing to warming trends. Understanding the dynamics of ice formation and melt is essential for predicting future climate change scenarios and mitigating its impacts.
Conclusion
The phenomenon of ice floating in cold water is a captivating example of how seemingly simple observations can reveal complex scientific principles. Through the interplay of density, buoyancy, temperature, and unique properties of water, we gain a deeper understanding of the natural world around us.
This intricate dance between ice and water shapes our planet’s ecosystems, influences global climate patterns, and reminds us of the interconnectedness of all things in nature.